QUESTION
What is meant by disproportionation? Give two examples of disproportionation reaction in aqueous solution
ANSWER
In a disproportionation reaction, same substance undergoes oxidation (increase in oxidation number) as well as reduction (decrease in oxidation number) resulting in the formation of two different products.e.g.
(i) Mn (VI) becomes unstable relative to Mn (VII) and Mn
(IV) in acidic solution.
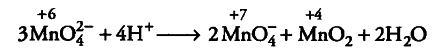
(ii) Many Cu (I) compounds are unstable in aqueous solution and undergo disproportionation as follows:
![]()
QUESTION
Do the vital functions of the body such as digestion get affected during fever? Explain your answer.
ANSWER
The optimum temperature range for enzymatic activity is 298-310 K, i.e. enzymes are inactive beyond this temperature range (high or low).
Thus, during fever (temperature >310 K), the activity of enzymes get affected.


 0
0

