| The first law of thermodynamics is the application of the conservation of energy principle to heat and thermodynamic processes: 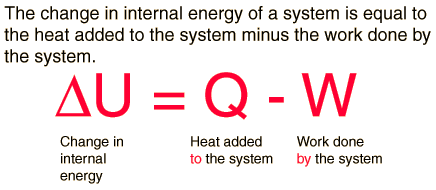 The first law makes use of the key concepts of internal energy, heat, and system work. It is used extensively in the discussion of heat engines. The standard unit for all these quantities would be the joule, although they are sometimes expressed in calories or BTU. It is typical for chemistry texts to write the first law as ΔU=Q+W. It is the same law, of course - the thermodynamic expression of the conservation of energy principle. It is just that W is defined as the work done on the system instead of work done by the system. In the context of physics, the common scenario is one of adding heat to a volume of gas and using the expansion of that gas to do work, as in the pushing down of a piston in an internal combustion engine. In the context of chemical reactions and process, it may be more common to deal with situations where work is done on the system rather than by it.
|
System WorkWhen work is done by a thermodynamic system, it is ususlly a gas that is doing the work. The work done by a gas at constant pressure is:
For non-constant pressure, the work can be visualized as the area under the pressure-volume curve which represents the process taking place. The more general expression for work done is: 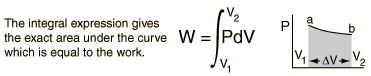 Work done by a system decreases the internal energy of the system, as indicated in the First Law of Thermodynamics. System work is a major focus in the discussion of heat engines. |


 0
0



